News
Scientists Use U.S. NSF Supercomputers to Reveal How “Jumping Genes” can Transform Gene Editing Technologies
Published September 18, 2025
By Kimberly Mann Bruch
University of California Riverside (UCR) researchers recently utilized U.S. National Science Foundation (NSF) ACCESS allocations to uncover how a bacterial protein can steer the “jumping” of genes to specific spots in the genome. Think of it as upgrading genetic scissors into a tool that can also paste in new instructions exactly where needed. These mobile bits of DNA, called transposons, could one day let scientists add beneficial genes with pinpoint accuracy, but how to guide their jumps remains a mystery.
Scientists say that understanding how this system works could pave the way for transformative gene-editing tools that go beyond simply cutting out genes — enabling the precise insertion of helpful genes for applications ranging from medicine to agriculture.
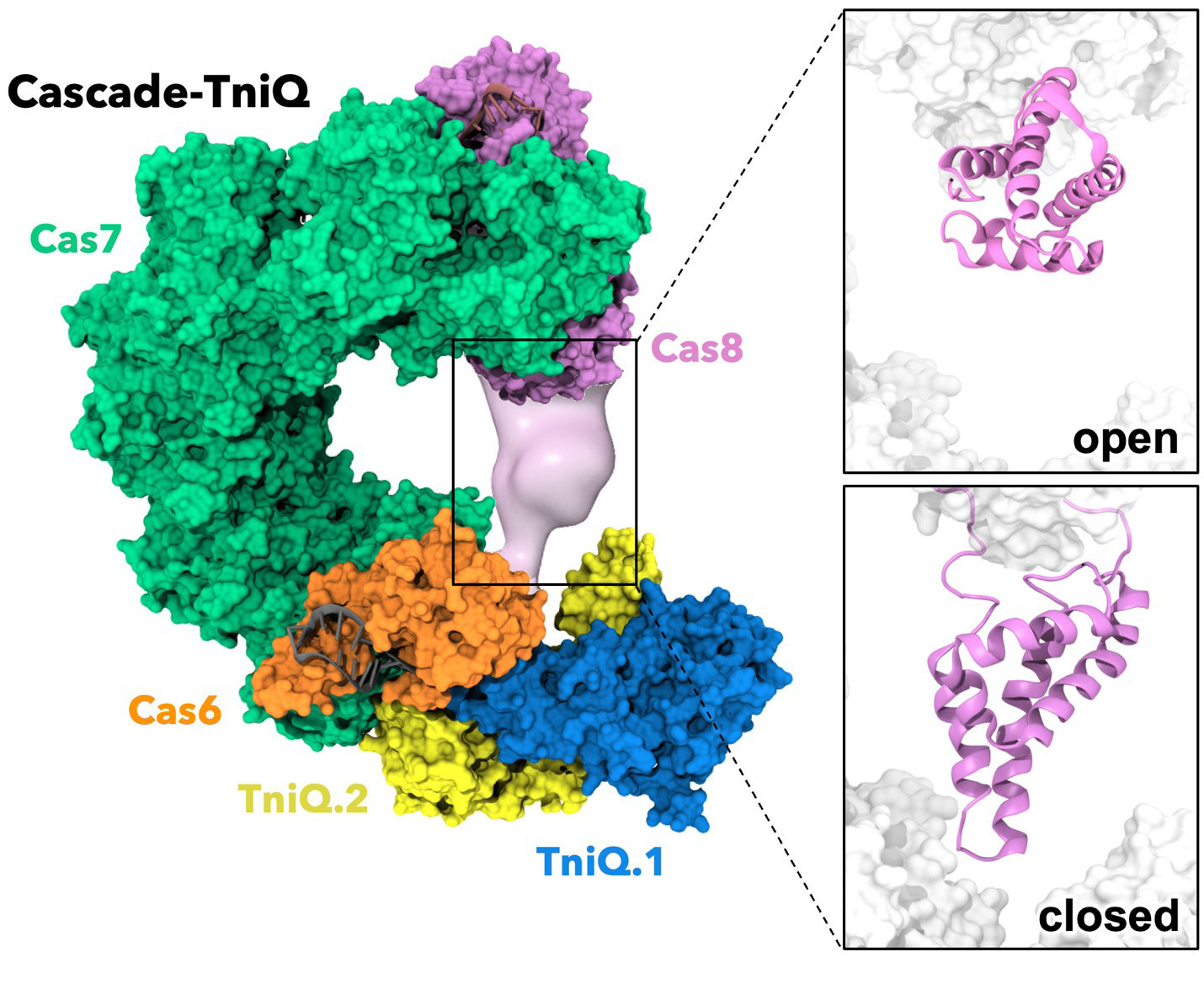
Using the Expanse system at the San Diego Supercomputer Center (SDSC), which is a part of the School of Computing, Information and Data Sciences at University of California San Diego and Bridges-2 at the Pittsburgh Supercomputing Center (PSC), researchers from Giulia Palermo’s lab explored a majestic protein/nucleic acid complex from cholera bacteria called Cascade-TniQ. One key part, a protein named Cas8, works like a flexible hinge capable of opening to bind DNA once the target sequence is detected.
“Nobody really knew how this molecular machine moves and changes shape to bind DNA, nor coordinate all its parts,” said Amun C. Patel, a graduate student in the UCR Bioengineering Department, the multidisciplinary biophysics program and member of the Palermo lab. “It was like knowing a sophisticated robot could perform surgery but having no idea how its joints and sensors worked together to do so.”
Patel worked with the SDSC team and collaborated with Souvik Sinha and Pablo R. Arantes in the Palermo lab — co-authors of the study published in Proceedings of the National Academy of Sciences — to run advanced simulations on SDSC’s Expanse and PSC’s Bridges-2 supercomputers.
“We built detailed models of this large complex and discovered that the bacterial system works in two main states. Cas8 can be closed, like an off switch — or open and ready to bind DNA,” Patel explained. “When the system detects the right DNA sequence, Cas8 begins to open. Once the matching DNA attaches, Cas8 fully opens, exposing the chemical surfaces needed to grab onto the DNA and complete the binding process. This ‘turns on’ the Cascade-TniQ machine so it can recruit the downstream cutting-pasting mechanisms characteristic of transposons.”
Patel said that what makes this complex so remarkable is its finely tuned coordination. The simulations revealed that Cas8’s movements are tightly synchronized with the transposition proteins TniQ. If either Cas8 or TniQ fails to move or orient correctly, the entire molecular machine stalls. Even more striking, TniQ is the “conductor” that brings in the proteins needed for the cut-and-paste process. This means both parts of the Cascade–TniQ system – the Cascade complex and TniQ – must work in perfect harmony for gene insertion to succeed, making this transposition event an exquisitely precise and highly reliable process.
“It's like upgrading from basic scissors to a precision surgical instrument that can install new parts,” she said. “And this work would not have been possible without the NSF ACCESS allocations on Expanse at SDSC and Bridges-2 at PSC.”
Funding was provided by the U.S. National Institutes of Health (grant no. R01GM141329), the U.S. NSF (grant no. CHE-2144823), the Alfred P. Sloan Foundation (grant no. FG-2023-20431) and the Camille and Henry Dreyfus Foundation (grant no. TC-24-063). Computational studies using the Expanse system at SDSC was funded by NSF ACCESS (allocation no. MCB160059) while computational work on Bridges-2 at PSC was funded by NSF ACCESS (allocation no. BIO230007).

