News
Scientists Use Advanced Microscopy to Reveal How Isocyanide Sticks to Metal Surfaces
Published May 30, 2025
By Kimberly Mann Bruch
A UC San Diego research team has utilized U.S. National Science Foundation (NSF) ACCESS allocations on Expanse at the San Diego Supercomputer Center (SDSC) – part of the School of Computing, Information and Data Sciences (SCIDS) – to better understand how a molecule called isocyanide bonds to different metals. Commonly used in industrial chemistry applications such as the development of electronic devices, isocyanide is often thought of as possibly useful, yet too toxic to carefully study. However, UC San Diego scientists developed a powerful way to observe exactly how individual isocyanide molecules attach to metal surfaces – in a safe manner. Their findings revealed surprising details that could help engineers design better catalysts and electronic components.
The team’s comprehensive study, which used simulations on SDSC’s Expanse in conjunction with ultra-high-resolution microscopy with vibrational spectroscopy, has been published in Nano Letters.
“These isocyanide molecules contain a carbon-nitrogen bond that acts like a molecular ‘fingerprint’ – its vibration frequency changes depending on how the molecule bonds to different metals,” said Shaowei Li, an assistant professor in UC San Diego’s Department of Chemistry and Biochemistry. "Think of it like a guitar string where the same string will produce different musical notes depending on how tightly it's stretched – similarly, when these molecules bind to metal surfaces in different ways, their chemical bonds vibrate at different frequencies."
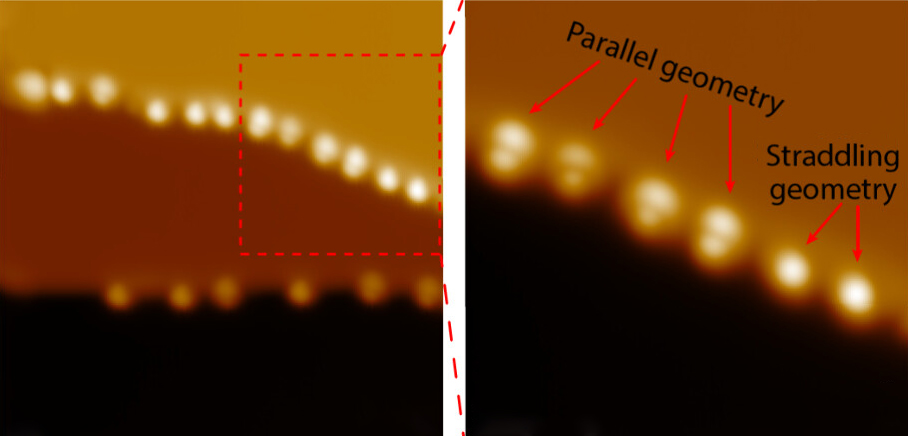
Previous methods for studying these interactions were like trying to listen to individual instruments in an orchestra from far away – scientists could detect the overall chemical signature but couldn't pinpoint exactly how individual molecules were positioned. The new technique, called inelastic electron tunneling spectroscopy with scanning tunneling microscopy (STM-IETS), is like having a microscopic ear that can listen to each molecule separately.
Using this approach, the researchers examined isocyanides attached to copper and silver surfaces. They discovered that the precise geometry of how a molecule sits on a surface has a much bigger impact on its vibrational signature than previously thought – even more significant than switching between completely different metals.
“Our findings challenge conventional assumptions about molecule-metal interactions and could lead to more precise control in manufacturing processes that rely on these relationships, such as catalytic converters in cars or the production of pharmaceuticals,” Li said. “This new molecular-level understanding represents a step toward designing materials with tailored properties by controlling exactly how molecules arrange themselves on surfaces – we could not have accomplished this without funding from the National Science Foundation and the Department of Energy.”
The use of facilities and instrumentation for this research was supported by the NSF through the UC San Diego Materials Research Science and Engineering Center (grant no. DMR-2011924) with primary support provided by the U.S. Department of Energy (grant no. DE-SC0025537) and NSF (grant no. DMR-2011924, CHE-2303936 and DMR-2106713. Computational resources on Expanse were funded by ACCESS (allocation no. CHE240205).
Additional research conducted by the team has been published in the Journal of the American Chemical Society.

